WORKING ON DISCOVERING DISEASE GENES AND UNDERSTANDING THE PATHOLOGICAL PROCESSES IN NEUROMUSCULAR DISORDERS.
Neuromuscular diseases constitute the largest genetic group of diseases affecting children and adults resulting in impaired locomotion in affected patients. Clinically, they form a highly heterogeneous group of diseases ranging from severe congenital forms to a mild childhood form with survival into adulthood. Gene discovery in rare forms of the disease is extremely challenging due to clinical and genetic heterogeneity and small sample sizes. In past few years, we have discovered several disease-causing genes using a combination of next generation sequencing approaches and functional modeling in animal models. We work closely with national and international clinicians and researchers to accelerate the gene discovery in neuromuscular diseases.
a. GOLGA2, encoding a master regulator of golgi apparatus, is mutated in a patient with a neuromuscular disorder (2016). Shamseldin HE, Bennett AH, Alfadhel M, Gupta VA*, Alkuraya F*. Hum. Genet. PMID:26742501 (*Co-corresponding authors)
b. LMOD3 is an essential regulator of actin thin filaments in skeletal muscle (2014). Yuen M, Sandaradura SA, Dowling JJ, Kostyukova AS, Moroz N, Quinlan KG, Lehtokari V-L, Ravenscroft G, Todd EJ, Ceyhan-Birsoy O, Gokhin DS, Maluenda J, Lek M, Nolent F, Pappas CT, Novak SM, D’Amico A, Malfatti E, Thomas BP, Gabriel SB, Gupta N, Daly MJ, Ilkovski B, Houweling PJ, Swanson LC, Brownstein CA, Gupta VA, Medne L, Shannon P, Martin N, Bick DP, Flisberg A, Holmberg E, Bergh PV, Lapunzina P, Waddell LB, Sloboda DD, Bertini E, Chitayat D, Telfer WR, Laquérriere A, Gregorio CC, Ottenheijm C, Bönnemann CG, Pelin K, Beggs AH, Hayashi YK, Melki J, Romero NB, Laing NG, Nishino I, Wallgren-Pettersson C, Fowler VM, MacArthur DG, North KN, Clarke NF. J Clin. Invest., PMID: 25250574
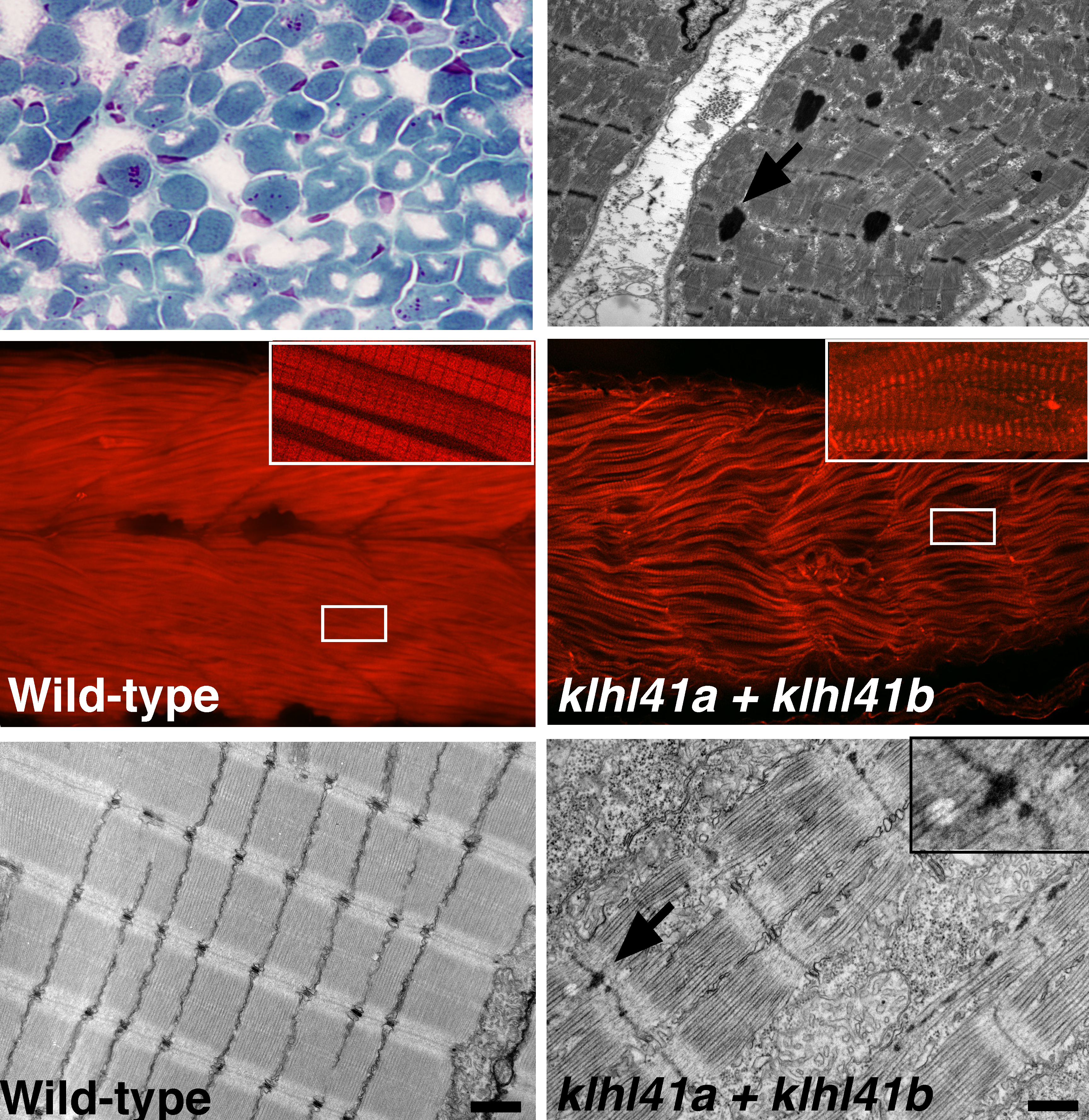
c. Identification of KLHL41 mutations implicates BTB-Kelch-mediated ubiquitination as an alternate pathway to myofibrillar disruption in nemaline myopathy (2013). Gupta VA, RavenscroftG, ShaheenR, ToddEJ, SwansonLC, ShiinaM, OgataK, HsuC, ClarkeNF, DarrasBT, FarrarM, HashemA, Manton N , MuntoniF, NorthKN, SandaraduraS, NishinoI, HayashiYK, SewryCA, ThompsonE, YuTW, BrownsteinCA, AllcockR, DavisMR, Wallgren-PetterssonC, MatsumotoN, AlkurayaFS, LaingNG, BeggsAH. Am. J. Hum. Genet., PMID: 24268659
d. Whole-exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause for Walker-Warburg Syndrome (2012). Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, HeinzenEL, Shianna KV, Stevens CR, Partlow JN, Barry BJ, Rodriguez J, Gupta VA, Al-Qudah A-K, Eyaid WM, Friedman JM, Salih MA, Clark R, Moroni I, Mora M, Beggs AH, Gabriel SB, Walsh CA. Am. J. Hum. Genet.: PMID: 22958903
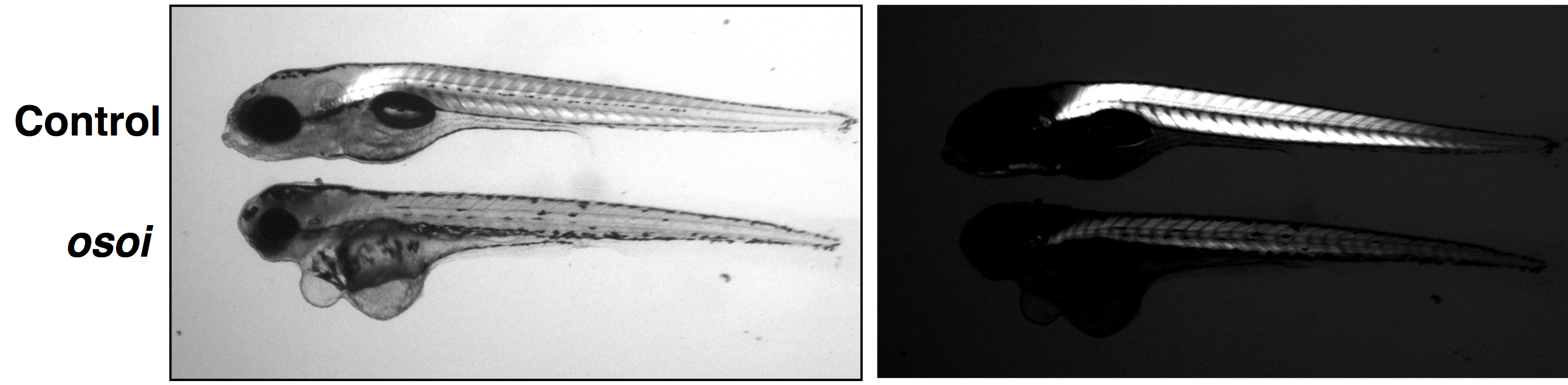
The normal functioning of neuromuscular development requires a complex coordination of skeletal muscles, neuromuscular junctions and motor or sensory neurons. Molecularly, these processes are regulated by both extrinsic and intrinsic factors. To identify these factors and study their contribution to disease pathogenesis in a physiological milieu, we are using forward and reverse genetic approaches in small animal models. With this approach, we have identified several new factors and biological processes regulating disease pathology as well as delineated the roles of known disease genes. Together, these approaches will help to identify novel targets for therapeutic interventions in neuromuscular diseases.
a. Mutation-specific effects on thin filament length in nemaline myopathy (2016). de Winter JM, Joureau B, Lee EJ, Kiss B, Yuen M, Gupta VA, Pappas CT, Gregorio CC, Stienen GJM, Edvardson S, Wallgren-Pettersson C, Lehtokari VL, Pelin K, Malfatti E, Romero NB, van Engelen BG, Voermans NC, Donkervoort S, Bönnemann CG, Clarke NF, Beggs AH, Granzier H, Ottenheijm CAC. Annals of Neurology. PMID: 27074222
b. Fam65b is important for formation of the HDAC6-dysferlin protein complex during myogenic cell differentiation (2014). Balasubramanian A, Kawahara G, Gupta VA, Rozkalne A, Beauvais A, Kunkel LM, Gussoni E. FASEB J. PMID: 24687993
c. Loss of catalytically inactive lipid phosphatase myotubularin-related protein 12 impairs myotubularin stability and promotes centronuclear myopathy in zebrafish (2013). Gupta VA, Hnia K, Smith LL, Gundry SR, McIntire JE, Shimazu J, Bass JR, Talbot EA, Amoasii L, Goldman NE, Laporte J, Beggs AH. PLoS Genet. PMID: 23818870
d. Myotubularin-deficient myoblasts display increased apoptosis, delayed proliferation, and poor cell engraftment (2012). Lawlor MW, Alexander MS, Viola MG, Meng H, Joubert R, Gupta VA, Motohashi N, Manfready RA, Hsu CP, Huang P, Buj-Bello A, Kunkel LM, Beggs AH, Gussoni E. Am J Pathol. PMID:22841819
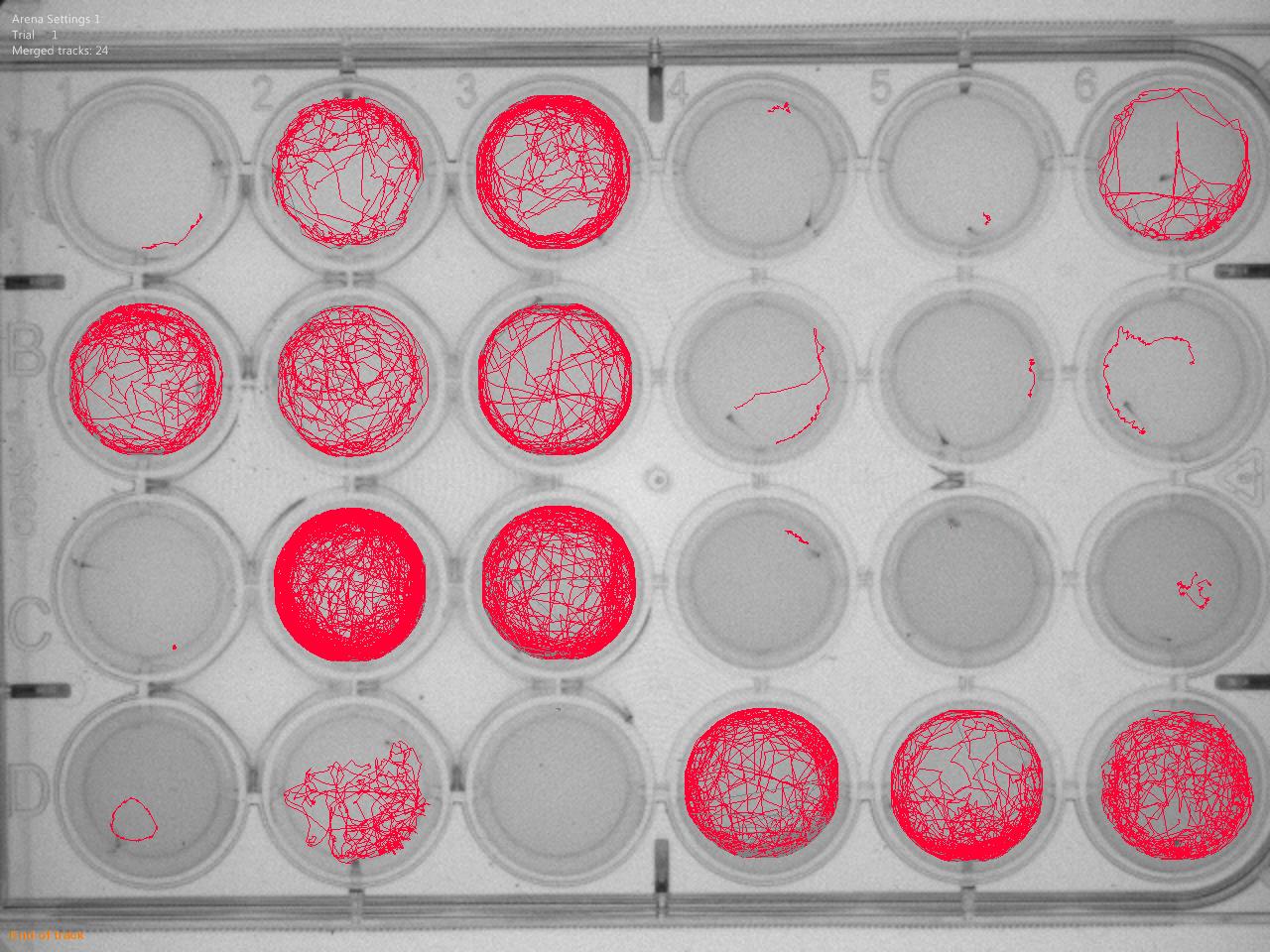
The long-term goal of our laboratory is to develop novel regenerative medicine approaches for restoring normal mobility in patients affected with neuromuscular diseases. We are using chemical genetic approaches in cellular and animal models to identify small molecule regulators that can promote skeletal muscle growth and repair processes.